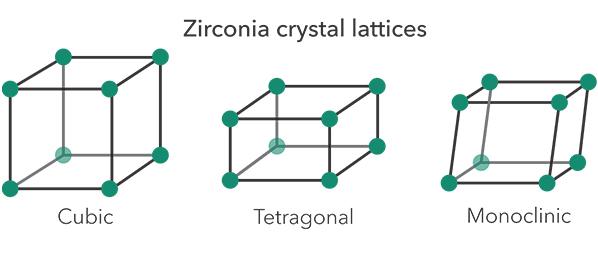
The three phases of zirconia
Zirconia or zirconium oxide (ZrO2) exists in various types, based on the yttria content and uniform or hybrid composition of the crystal lattice.
The different types of zirconia are built from three different ‘phases’, each with its own crystal structure or lattice. Zirconia adopts a monoclinic crystal structure at room temperature and transitions to tetragonal and cubic at higher temperatures. Phase transitions from monoclinic to tetragonal to cubic phase induce volumetric changes.
Monoclinic zirconia has low strength and translucency. Tetragonal and cubic zirconia exist at room temperature when stabilised with other oxides, called ‘dopants’. Dopants include yttrium oxide (Y2O3, yttria) and calcium oxide (CaO). Minor amounts of aluminum oxide (Al2O3, alumina) are also often added.
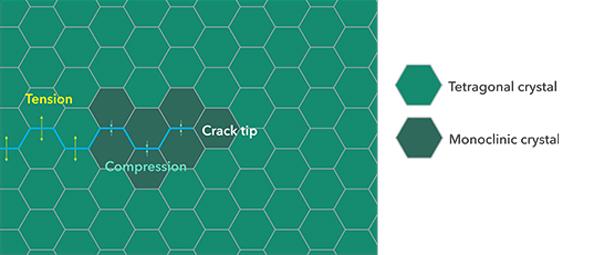
Transformation toughening
Toughness of zirconia
Tetragonal zirconia is extremely tough, owing to a unique process of transformation toughening. When a crack starts to form, the tetragonal zirconia around the crack transforms to a monoclinic structure and hereby expands in propagation and enhances the toughness of the material. While it enhances the fracture toughness, repeated transformation cycles can lead to degradation over time, especially in a moist environment. This is the reason why sandblasting zirconia mustn’t be done at high pressure and grinding it after sintering should be avoided. This degradation process is mitigated by the alumina content.
Strength and translucency: a trade-off
Approximately 3 mol% yttria should be added to keep the material stable at room temperature; this variety is therefore sometimes described as 3Y zirconia or 3Y-TZP (tetragonal stabilised zirconia polycrystal; e.g. Initial Zirconia Disk HT).
Increased levels of yttria (typically 5Y-TZP with 5 mole % Y-TZP or more) and a higher proportion of cubic phase in zirconia result in higher translucency but reduce the strength (e.g. Initial Zirconia Disk UHT). Cubic zirconia does not exhibit transformation toughening and is more brittle than tetragonal zirconia, but stronger than monoclinic zirconia. Other dopants such as Al2O3 in very small amounts further influence the ZrO2 lattice.
Zirconia with lower yttria content (3Y-TZP, 3 mole % Y-TZP) has better mechanical properties and less translucency whereas an increased yttria content (5Y-TZP with 5 mole % Y-TZP) has more translucency but presents lower mechanical properties. Nevertheless, it’s still stronger than lithium disilicate!
Therefore, zirconia can be luted either conventionally (e.g. FujiCEM Evolve) or adhesively (e.g. G-CEM ONE, which contains MDP)*
* Discover more in our other blogs:
What about Vertiprep and BOPT: emerging restoration approaches
How much strength is required for my ceramic restorations? And how is this determined?
Tips for durable cementation
| Tetragonal | Tetragonal/cubic | Tetragonal/cubic |
|---|---|---|
|
100% Tetragonal |
~75% Tetragonal ~25% Cubic |
~50% Tetragonal ~50% Cubic |
|
3Y-TZP/0.25 Al2O3 |
4Y-TZP/0.05 Al2O3 “4th generation” |
5Y-TZP/0.05 Al2O3 “3rd generation” |
|
3Y-TZP/0.05 Al2O3 “2nd generation” |
5Y-TZP/0.02 Al2O3 “5th generation” |
Different forms of zirconia. Note that the number before the "Y" indicates the molar percentage of yttria percentage and not the generation.
While the main types of zirconia are briefly explained here, it comes in many different forms and varieties. It’s important to remember that one zirconia is not the other, which needs to be kept in mind when making treatment decisions
- Chevalier J, Gremillard L, Virkar AV, Clarke DR. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J Am Ceram Soc. 2009;92(9):1901–1920.
- Belichko DR, Konstantinova TE, Volkova GK, Mirzayev MN, A.V. Maletsky AV, V.V. Burkhovetskiy VV, ADoroskevich AS, Mita C, Mardare DM, Janiska B, Nabiyev AA, Lyubchyk AI, Tatarinova AA, Popov E. Effects of YSZ ceramics doping with silica and alumina on its structure and properties. Mater Chem Phys. 2022;287:126237.

